
 Each agency should have a separate authorization and include the appropriate contacts from the respective agency. FDA Component Contact Name: Office of Oncologic Disease Division Director. Sponsor Authorization Letter (SAL) Template. Please contact the review division for most current template. Currently, Lauren manages applications with foreign regulatory counterparts under Project Orbis, facilitates international communication and assists with OCE internal planning for advisory committee meetings. She joined OCE and the Project Orbis team in 2020. She resumed her government career in 2014 as the Designated Federal Officer for the Oncologic Drugs and Antimicrobial Drugs Advisory Committees at the FDA. Prior to FDA, Lauren was an Assistant Professor of Pharmacy Practice at the South College School of Pharmacy in Knoxville, TN, and completed two years of post-graduate pharmacy training in infectious diseases at Lee Health in Fort Myers, FL. Lauren has been in government service since high school as an intern at the Center for Veterans Enterprise, an agency of the Department of Veteran Affairs (VA) and as a pharmacy technician during pharmacy school at the Washington, DC VA Medical Center. She holds a Doctor of Pharmacy degree from The Philadelphia College of Pharmacy, Philadelphia, PA, and board certification in Pharmacotherapy (2012) and Infectious Diseases from the Board of Pharmacy Specialties. Lauren Tesh Hotaki is a Senior Regulatory Health Project Manager in the OCE. Currently, Dianne facilitates OCE interactions with foreign regulatory counterparts and was instrumental in the first international collaborative review between Australia, Canada, and the US under Project Orbis. From late 2005-early 2019, she was the Lead Project Manager for the Oncology Program in the Office of Hematology and Oncology Products.
Each agency should have a separate authorization and include the appropriate contacts from the respective agency. FDA Component Contact Name: Office of Oncologic Disease Division Director. Sponsor Authorization Letter (SAL) Template. Please contact the review division for most current template. Currently, Lauren manages applications with foreign regulatory counterparts under Project Orbis, facilitates international communication and assists with OCE internal planning for advisory committee meetings. She joined OCE and the Project Orbis team in 2020. She resumed her government career in 2014 as the Designated Federal Officer for the Oncologic Drugs and Antimicrobial Drugs Advisory Committees at the FDA. Prior to FDA, Lauren was an Assistant Professor of Pharmacy Practice at the South College School of Pharmacy in Knoxville, TN, and completed two years of post-graduate pharmacy training in infectious diseases at Lee Health in Fort Myers, FL. Lauren has been in government service since high school as an intern at the Center for Veterans Enterprise, an agency of the Department of Veteran Affairs (VA) and as a pharmacy technician during pharmacy school at the Washington, DC VA Medical Center. She holds a Doctor of Pharmacy degree from The Philadelphia College of Pharmacy, Philadelphia, PA, and board certification in Pharmacotherapy (2012) and Infectious Diseases from the Board of Pharmacy Specialties. Lauren Tesh Hotaki is a Senior Regulatory Health Project Manager in the OCE. Currently, Dianne facilitates OCE interactions with foreign regulatory counterparts and was instrumental in the first international collaborative review between Australia, Canada, and the US under Project Orbis. From late 2005-early 2019, she was the Lead Project Manager for the Oncology Program in the Office of Hematology and Oncology Products. 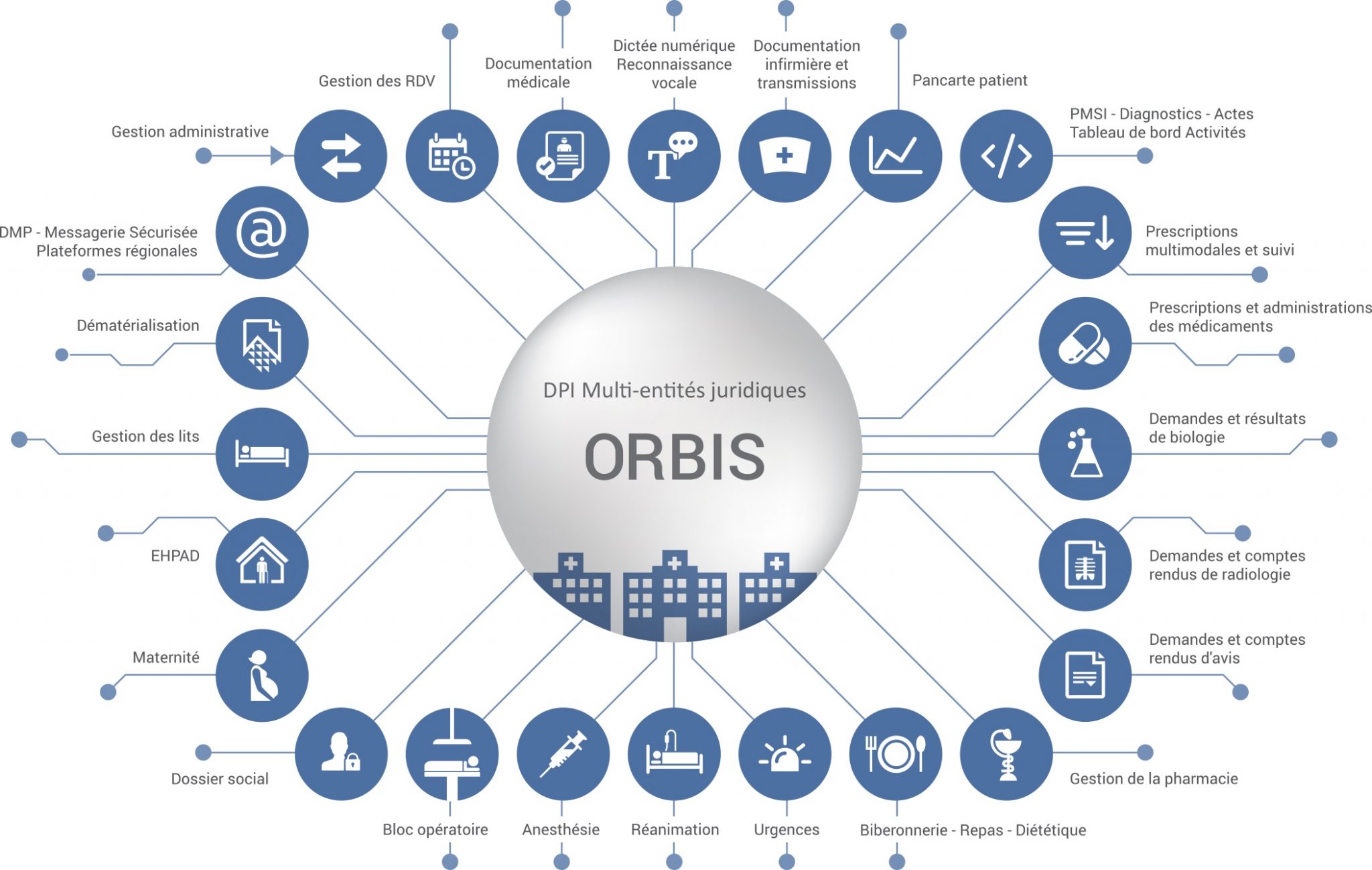
Richard Pazdur, then the Director of the Division of Oncology Drug Products. Between 2002-2005, she served as Special Assistant to Dr.

In 1993, she joined the FDA/CDER Division of Oncology and Pulmonary Drug Products as a project manager. Dianne started in 1990 as a research assistant with the Veterinary Research Program at the National Center for Research Resources, National Institutes of Health. Her government career spans 30 years, with 27 of those years with FDA oncology Divisions and Offices. She holds a Bachelor of Science in Biology from Northeastern University, Boston, MA. Learn more about Project Orbisĭianne Spillman is the Associate Director for Global Regulatory Outreach in the Oncology Center of Excellence. For more information, please refer to the Project Orbis approval report. Since its inception, Project Orbis has received many oncology marketing applications and led to the approval of numerous oncology drugs for patients across the world.

Future drug development may benefit by establishing a greater uniformity of new global standards of treatment, leading to the optimal design of these important trials. Pivotal clinical trials in oncology are commonly conducted internationally and these global trials are increasingly important for investigating the safety and effectiveness of cancer drugs for approval in the United States. The FDA Oncology Center of Excellence (OCE) initiated Project Orbis in May 2019 to provide a framework for concurrent submission and review of oncology products among international partners.Ĭollaboration among international regulators may allow patients with cancer to receive earlier access to products in other countries where there may be significant delays in regulatory submissions, regardless of whether the product has received FDA approval.








 0 kommentar(er)
0 kommentar(er)
